INDIANAPOLIS — Here are Tuesday's latest updates on the coronavirus pandemic, including the latest news on COVID-19 vaccinations and testing in Indiana.
Registrations for the vaccine are now open for Hoosiers 12 and older through the Indiana State Department of Health. This story will be updated over the course of the day with more news on the COVID-19 pandemic.
State reports 2,378 new COVID cases, 89 additional deaths
The Indiana State Department of Health reported Tuesday that 2,378 more Hoosiers have tested positive for COVID-19. That brings the total confirmed case count in the state to 956,548 since March 2020.
ISDH also reported 89 new deaths from the period of Sept. 21-27. Indiana has lost 15,069 residents since the pandemic began.
The state also reported that 5,969 more Indiana residents have been fully vaccinated against COVID-19 as of Tuesday morning. The total number of Hoosiers now considered fully vaccinated is 3,254,662.
Latest US, world numbers
There have been more than 43.11 million confirmed cases of COVID-19 in the United States as of 3:30 a.m. Tuesday, according to Johns Hopkins University. There have been more than 690,400 deaths recorded in the U.S.
Worldwide, there have been more than 232.35 million confirmed coronavirus cases with more than 4.75 million deaths. More than 6.12 billion vaccine doses have been administered worldwide.
For most people, the coronavirus causes mild or moderate symptoms. For some, especially older adults and people with existing health problems, it can cause more severe illness like pneumonia, or death.
Elementary school-aged children could get Pfizer's COVID-19 vaccine by late October
Pfizer CEO Albert Bourla said the company plans to apply in just days for emergency use authorization of its COVID-19 vaccine for children ages 5-11.
Right now, the Pfizer vaccine is only approved for kids 12 and older. The American Academy of Pediatrics reports that children make up more than a quarter of all COVID-19 cases reported across the country.
Pfizer said pediatric trials showed that its COVID-19 vaccine is safe and effective for children ages 5-11. The emergency use authorization process with the Food and Drug Administration could begin this week. Shots could start going in the arms of younger children by the end of October.
"This is definitely a game-changer, not only in the sense of a pandemic, but if this vaccine is as effective in children as we've seen in adults, then this will definitely help us alleviate that from the challenges that we're experiencing, right - not only for schools and for public health, but for moms and dads as well,” said Thomas Duszynski, director of epidemiology education at the Fairbanks School of Public Health at IUPUI.
A vaccine for children ages 5-11 would cover most elementary school students. Vaccinated students relieve some of the hassle and burden of contact tracing for schools. Students who are vaccinated and show no symptoms can avoid the quarantines that have disrupted so many classrooms in the past couple months.
Pfizer said this dose for younger children is one-third the size of that given to people 12 and older.
Children ages 5-11 would receive two doses of the Pfizer vaccine, 21 days apart.
Pfizer reported that younger children in vaccine trials had comparable antibody responses and side effects as people ages 16-25. Pfizer is the only company with a vaccine ready for approval for children under 12, and the only vaccine already approved for children 12-17.
Hospitals fear staffing shortages as vaccine deadlines loom
Hospitals and nursing homes around the country are bracing for worsening staff shortages as state deadlines arrive for health care workers to get vaccinated against COVID-19.
With ultimatums taking effect this week in states like New York, California, Rhode Island and Connecticut, the fear is that some employees will quit or let themselves be fired or suspended rather than get the vaccine.
“How this is going to play out, we don’t know. We are concerned about how it will exacerbate an already quite serious staffing problem,” said California Hospital Association spokesperson Jan Emerson-Shea, adding that the organization “absolutely” supports the state's vaccination requirement.
New York health care employees had until the end of the day Monday to get at least one dose, but some hospitals had already begun suspending or otherwise taking action against holdouts.
About a dozen states have vaccination mandates covering health care workers in hospitals, long-term care facilities or both. Some allow exemptions on medical or religious grounds, but those employees often must submit to regular COVID-19 testing.
States that have set such requirements tend to have high vaccination rates already. The highest rates are concentrated in the Northeast, and the lowest ones in the South and Midwest.
The Biden administration also will require the roughly 17 million workers at health facilities that receive federal Medicare or Medicaid to be fully vaccinated under a rule still being developed.
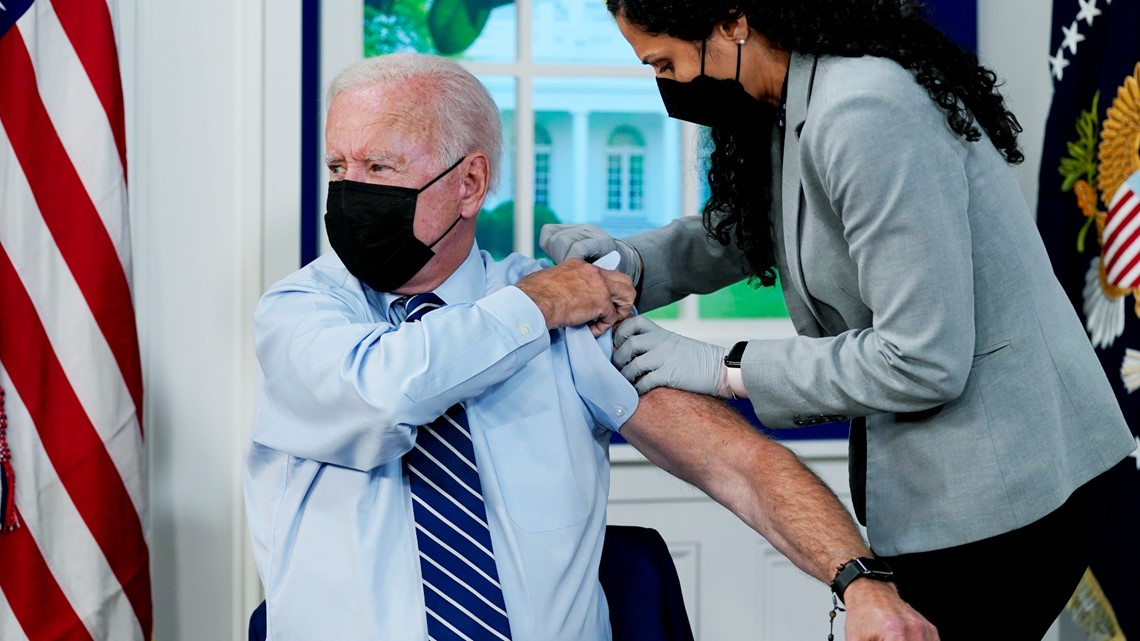
President Biden gets COVID-19 booster shot after authorization
President Joe Biden got his COVID-19 booster shot on Monday as part of the administration's efforts to encourage Americans to get vaccinated.
During brief remarks before receiving his booster shot at the White House, the president said that boosters are important, but the key is getting more people vaccinated.
Biden once again declared the situation has become a "pandemic of the unvaccinated."
Last week, federal regulators recommended a third dose of the Pfizer vaccine for Americans age 65 or older and approved them for others with preexisting medical conditions and high-risk work environments.
Biden got his first shot on Dec. 21 and his second dose three weeks later, on Jan. 11, along with his wife, Jill Biden. The president said the first lady was not getting her booster shot at the same time as him because she was teaching Monday.